Affinity Binding of Glycated Protein
Glycated haemoglobins (GHb) and glycated plasma proteins (GPP) differ from non-glycated proteins by the attachment of a sugar moiety(s) at various binding sites by means of a ketoamine bond. GHb and GPP thus contain 1,2-cis-diol groups not found in non-glycated proteins, which provide the basis for separation of glycated and non-glycated components by boronate affinity chromatography.1, 2
In this analytical technique, a boronate such as phenylboronic acid is bonded to the surface of the column support. When a solution of proteins (hemolysate or diluted plasma) is passed through the column, the glycated component is retained by the complexing of its diol groups with the boronate, as illustrated below. After the unretained non-glycated component elutes from the column, the glycated component is eluted from the column with a reagent that displaces it from the boronate.1,4
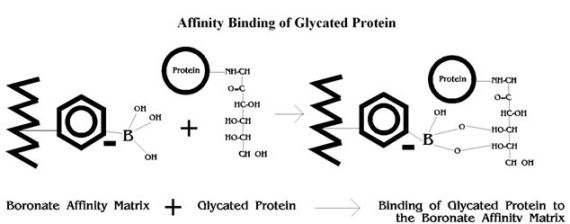
Boronate Affinity HPLC
Trinity Biotech’s Premier Hb9210 instruments employ the principles of boronate affinity and high-performance liquid chromatography (HPLC). The analytical column contains aminophenylboronic acid bonded to a porous polymer support (gel) and pumps transfer reagents & patient samples through the analytical column.
Hemolyzed samples for GHb are automatically injected onto the column during the flow of Elution Reagent #1 (Buffer 2A). The glycated component binds to the boronate, while the non-glycated component passes through the column to the spectrophotometric detector, where it is detected at 413 + 2 nm.
After the elution of the non-glycated component, the instrument pumps Elution Reagent #2 (Buffer 2A), which displaces the glycated component from the column. The glycated component then passes through the detector.
See the following diagram of the binding of glycated hemoglobin in the Affinity system.
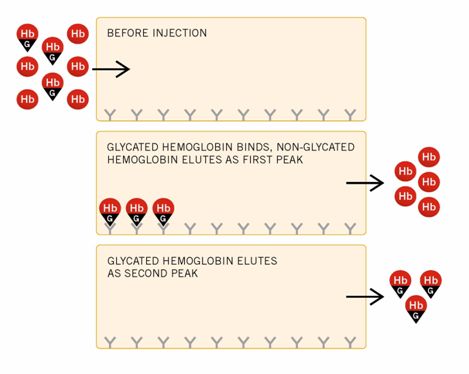
The compositions of Elution Reagents #1 and #2 are designed to exhibit virtually identical absorption in the 413 + 2nm range to ensure a stable baseline. The detector signal is also referenced by the split-beam technique. In the final stage of the cycle, the column is re-equilibrated with Elution Reagent #1. All reagent selection occurs in a timed sequence designed to allow complete elution of non-glycated and glycated components.
Boronate Affinity & HbA1c
All functions are controlled by the computer, which processes the signal from the spectrophotometric detector and calculates the concentration of glycated hemoglobin or plasma protein as a percentage of the total detected. Integration is by peak area in Absorbance Units (AU)-seconds. The computer produces printed reports by analyzing signal as it is received by the detector. A Batch Summary Report is printed at the end of the run.
The software, especially designed for GHb/HbA1c analysis, controls the four basic system operation functions of sample identification, instrument operation, calculation of results and printing (and storing) complete reports. Based on computer hard disc capacity, many years worth of data (chromatograms and batch summary reports) can be automatically archived.
Calculation of the percentage of GHb in the sample is by the following formula, with peak area in AU/seconds:
![]()
The final result is obtained by comparison to reference samples, traceable to both NGSP & IFCC, using a 2-point calibration.
Benefits of Boronate Affinity for HbA1c
GHb measurement by boronate affinity chromatography is free from common interferences such as hemoglobin variants, non-glycation modifications and storage-related hemichromes.5 Boronate affinity chromatography requires no sample pretreatment to remove the labile (aldimine or Schiff base) component, since only stable (ketoamine-linked) GHb is retained by the boronate. Compared to ion-exchange techniques, affinity separation is also less sensitive to quantitative errors caused by minor fluctuations in reagent pH and ionic strength.2, 3 The system is temperature-controlled for maximum precision and provides faster, less expensive testing than the laborious thiobarbituric acid assay.
References
- Mallia, A. K. et al. “Preparation and use of a boronic acid affinity support for separation and quantitation of glycosylated hemoglobins.” Anal Lett 14:649-661 (1981)
- Fairbands, V. F. and Zimmerman, B. R. “Measurement of glycosylated hemoglobin by affinity chromatography,” Mayo Clin Proc 58:770-773 (1983)
- John, W. G. et al. “Affinity chromatography method for the measurement of glycosylated hemoglobin: comparison with two methods in routine use.” Clin Chim Acta 135:257-262 (1984)
- Willey, D. G. et al. “Glycosylated haemoglobin and plasma glycoprotein assays by affinity chromatography.” Diabetologia 27:56-58 (1984)
- Fluckiger, R. “Glycated Haemoglobins – Review.” J Chromatog 428:279-292 (1988)
There are many variations of passages of Lorem Ipsum available, but the majority have suffered alteration in some form, by injected humour, or randomised words which don’t look even slightly believable. If you are going to use a passage of Lorem Ipsum, you need to be sure there isn’t anything embarrassing hidden in the middle of text. All the Lorem Ipsum generators on the Internet tend to repeat predefined chunks as necessary, making this the first true generator on the Internet. It uses a dictionary of over 200 Latin words, combined with a handful of model sentence structures, to generate Lorem Ipsum which looks reasonable. The generated Lorem Ipsum is therefore always free from repetition, injected humour, or non-characteristic words etc.